Abstract
Background
In MDS, most risk assessment tools such as the IPSS-R estimate risk at time of diagnosis. Risk assigned by the IPSS-R categories, however, is not constant over time. Changes in hazard (= time specific risk in a short time interval) during course of the disease affect the use of prognostic tools and may influence clinical decisions. We have previously shown time related hazard changes in untreated patients leading to declining risk in higher risk groups and diminution of differences between risk groups over time. Aim of this study was to investigate hazard changes in patients undergoing disease-modifying treatment.
Patients and methods
16 international partners of the IWG-PM contributed data from 9179 primary MDS patients of which 2015 had disease modifying treatment: median age 70.0 yrs, 60% male, median follow up 4.1 yrs, median OS 3.8 yrs, IPSS-R categories: very low 16%, low 35%, intermediate 22%, high 15%, very high 12%. Treatment modalities included hypomethylating agents, lenalidomide, cytotoxic therapy, 17% had stem cell transplantation. Time variations were described by smoothed hazard graphs and by the Cox zph test including rho, a measure for a linear time dependent impact of a feature on hazard. Semiparametric accelerated failure time models were estimated by direct optimization procedures.
Results and discussion
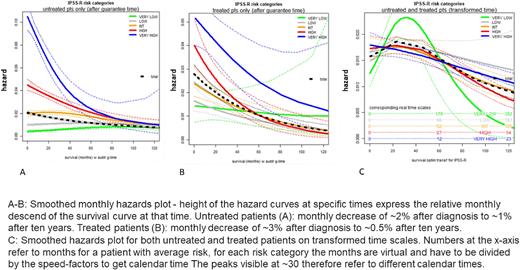
Using hazard graphs and the Cox zph-test we confirmed changes in hazard relations over time in the untreated subsample (rho -0.20 for OS). In treated patients a similar diminution was observed (rho -0.26 for OS). Results from treated patients revealed temporary increased hazard after some months especially in higher risk patients. Possible reasons are decision to start treatment when hazard increases or transient risk by therapy-associated toxicity, as well as a "guarantee time bias", induced by delayed treatment start, which in treated patients implicitly guarantees survival until start of treatment. To account for the latter, analyses were repeated excluding "guarantee time” in treated patients, again showing hazard reduction in higher risk patients and diminution between risk categories: untreated: rho -0.20 for OS, treated: rho -0.08 (fig A-B). Differently developing changes in hazards over time contradict the proportional hazards assumption of the Cox model. Therefore, as an alternative we investigated accelerated failure time models - fitting semi parametric models for IPSS-R risk categories: results show that patients in different risk categories share similar course of hazards - only at different "speeds” (speed-factors excluding guarantee time to achieve average survival: VERY LOW 0.34, LOW 0.63, INT 1.15, HIGH 2.23, VERY HIGH 5.11.) Transforming time accordingly makes common patterns visible, as in figure C, where most risk categories show hazard peaks before reaching the time of their median survival.
Conclusions
In MDS, hazards are not constant over time, possibly slightly rising until around the category specific median survival times and later clearly falling, except for the very high risk category. Longitudinal changes of hazards are not proportional between IPSS-R categories, instead diminution of hazard proportions between risk categories is observed. To describe the development of hazard for different IPSS-R categories the concept of different "speeds” of the disease development may be appropriate. Clinicians need to be aware of the dynamics of hazards in MDS when counselling patients both treated or those receiving supportive care only. Risk assessment at time of diagnosis by the IPSS-R assigns average group differences but changes over time need to be taken into account. Our data suggest that median survival times for IPSS-R categories approximately express different speeds and also indicate the time, when survival hazards are declining.
Disclosures
Pfeilstocker:BMS: Research Funding. Tuechler:BMS: Research Funding. Fenaux:Novartis: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Garcia-Manero:BMS: Consultancy, Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Gilead Sciences: Research Funding; Acceleron Pharma: Consultancy; AbbVie: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Aprea: Honoraria. Germing:Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria. Luebbert:AbbVie: Honoraria; Astex: Honoraria; Janssen: Research Funding; Otsuka: Consultancy; Syros: Consultancy; Cheplapharm: Other: study drug. Miyazaki:Daiichi-Sankyo: Honoraria; Takeda: Honoraria; Otsuka Pharmaceutical: Honoraria; Pfizer: Honoraria; Kyowa-Kirin: Honoraria; Bristol-Myers: Honoraria; Chugai: Honoraria; SyinBio: Honoraria; Dainippon-Sumitomo Pharma: Honoraria, Research Funding; Astellas: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Nippon Shinyaku: Honoraria; Janssen Pharmaceutical: Honoraria; Celgene: Honoraria. Sanz:takeda: Honoraria; Abbvie Pharmaceuticals: Other: Advisor or review panel participant; Helsinn: Honoraria, Other: Advisor or review panel participant; Novartis Oncology: Consultancy; Janssen Pharmaceuticals, Inc.: Other: Teaching and Speaking; Celgene Corporation: Consultancy; La Hoffman Roche Ltd.: Other: Advisor or review panel participant; Takeda Pharmaceuticals Ltd: Other: Advisor or review panel participant. Santini:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Menarini: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Sekeres:Bristol Myers-Squibb: Membership on an entity's Board of Directors or advisory committees; Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kurome: Membership on an entity's Board of Directors or advisory committees. Valent:Blueprint Medicines: Honoraria; Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Incyte: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal